

Flow Cytometry and Microbiology
Activity of common oral antiseptics against bacteria
assessed using the oxonol DIBAC4(3).
F. Paul, R. Jepras, D. Hynes, A.Smith and B. Marken
Analytical Sciences
SmithKline Beecham Pharmaceuticals
Brockham Park, Betchworth
Surrey RH3 7AJ, UK
 email: Robert_Jepras-1@sbphrd.com
email: Robert_Jepras-1@sbphrd.com
Abstract
The activity of triclosan (TRI), chlorohexidine (CHX) and cetylpyridinium chloride (CPC),
commonly found in consumer healthcare products, against some strains of oral bacteria were
compared. A flow cytometry technique using the membrane potential probe
bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3)] was performed in parallel
with conventional CFU assays. By monitoring the mean fluorescence of Streptococcus mutans,
Streptococcus oralis and Streptococcus sanguis the activity of these compounds was demonstrated.
Comparison of flow cytometry viable counts showed a close correlation with the corresponding
CFU assays and on no occasion was an effect shown by CFU assay and not by flow cytometry.
Results were obtained in a few minutes using flow cytometry whereas information from standard
CFU assays took at least 24-48h. Flow cytometry gave information on the immediate effects of
the compounds, that is, their activity in the first few minutes which is more appropriate for
assessing the effectiveness of orally active compounds. The flow cytometry results indicated
that the activity of the compounds can be determined in most instances within the first 2 minutes
of treating the bacteria.
Introduction
There is an obvious requirement for the active ingredients used in oral care product such as
mouth wash and toothpaste to rapidly kill the bacteria that cause tooth decay and gum disease.
This is mainly because of the short exposure times and rapid dilution of active ingredients by
saliva. It follows, therefore, that methods that can quickly detect this early activity is
highly appropriate and beneficial in evaluating new compounds.
In this study some common biocides were compared for their activity against oral bacteria
using flow cytometry and standard CFU assays. They all show similarities in their activity
by acting on the cytoplasmic membrane. Consequently the membrane potential sensitive oxonol
DiBAC4(3) was used as an appropriate fluorescent viability probe(1).
Cetylpyridinium chloride (CPC) is a cationic quaternary ammonium compound and acts on the cell
membrane resulting in a generalized loss in its function as a permeability barrier(2).
Chlorhexidine (CHX) is a biguanide and causes the collapse of membrane potential via the
disruption of the membrane rather than ATPase activity(3). Triclosan
[5-chloro-2-(2,4-dichlrophenoxy phenol] (TRI) acts on the cytoplasmic membrane
and is bacteriostatic at low concentrations interfering with the uptake of nutrients(4).
Materials and methods
Culture samples were removed from their growth medium by centrifugation and washed 3 times in
0.22 um filtered PBS. The concentration was adjusted to approximately 1x107 organisms/ml.
DiBAC(4)3 (Molecular Probes. Inc.) was added to give a final concentration of 10 ug/ml. The
biocides were added to give a 5 x MIC for each of the microorganisms (see figure legends for
further details). Samples were removed at various times for CFU assays and flow cytometry
studies using a Bryte HS (Bio-Rad) flow cytometer. Excitation and emission wavelengths were
470-490 nm and 520-550 nm respectively.
Results and discussion
The typical biocide effects on bacteria are shown in Fig.1. It represents the flow cytometry
dot plot depicting DiBAC(4)3 fluorescence against forward angle light scattering from S. mutans
before and after 15 mins treatment with CHX. There are two distinct populations of cells
following treatment The denser of the two has a higher fluorescence intensity and represents
the dead or dyeing cells. The dead cell population has a much broader light scattering
distribution. Thus, indicating that the cell membrane or wall has been perturbed causing a
loss in membrane potential and a change in cell morphology. Viable counts can be determined
directly by placing regions of interest around the clusters of live cells.


Figure 1. DiBAC4(3) fluorescence (FL1) versus forward light scattering (LS1) dot plot of
S. mutans before (top) and after (bottom) treatment with 9 mg/ml chlorohexidine for 15 min.
Figs. 2,3 and 4 show the effects increasing biocide concentration after 30s treatment on the
mean fluorescence intensity of the microorganisms. There is very little difference in the
effects of each compound at lower concentrations, however, at concentrations above 12.5 ug/ml
CPC has the highest activity against all strains whereas TRI has the least. S. sanguis is the
least affected of the strains tested by all the compounds.
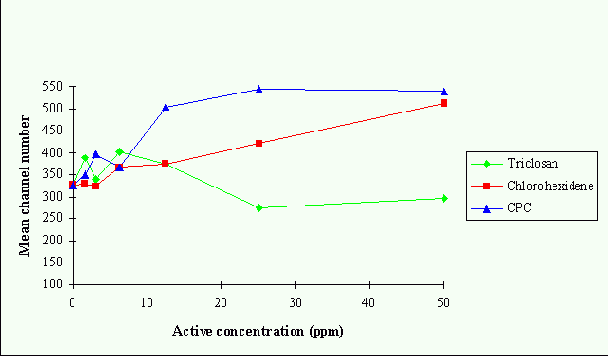
Figure 2. Effects of triclosan, chlorohexidine and cetylpyridinium chloride on DiBAC4(3)
fluorescence from S. mutans after 30s of treatment.

Figure 3. Effects of triclosan, chlorohexidine and cetylpyridininum chloride (CPC) on
DiBAC4(3) fluorescence from S. oralis after 30s treatment.

Figure 4. Effects of triclosan, chlorohexidine and cetylpyridininum chloride
on DiBAC4(3) fluorescence from S. sanguis after 30s treatment.
Conclusions
Flow cytometry used in combination with the membrane potential probe DiBAC4(3) offers several
advantages over standard CFU assays for determining the effectiveness of antibacterial compounds
used in oral care products. The immediate effects on bacteria can be detected, i.e., the actions
within 30s of exposure which may be a more relevant indicator of efficacy. At the same time
results are obtained the same day as the experiment. The acute effect of antibacterial compounds
is difficult to obtain using conventional microbiological methods which involve, serial
dilution's, plating and a 24-48h incubation period. The ability to obtain results quickly is
important particularly when screening new compounds and during QA tests. The use of the Flow
Cytometric assay will have particular usefulness during formulation experiments where a large
range and combination of active materials needs to be tested. The effect of other constituents
of oral care products could also be determined.
References
1) Jepras. R.I., Carter, J.H., Pearson, S.C., Paul, F.E. and Wilkinson, M.J. (1995).
Development of a robust flow cytometric assay for determining viable numbers of bacteria,
Applied and Environmental Microbiology, 61, 2696-2701.
2) Hugo,W.B. (1992). Disinfection mechanisms, In: (Russell, A.D., Hugo,W.B. and Ayliffe,
G.A.J. eds.), Principles and Practice of Disinfection, Preservation and Sterilisation.
2nd edn, pp. 180-186. Oxford: Blackwell Scientific Publications.
3) Russell, A.D. (1995). Biocides: Activity, Action and Resistance. In ( Hunter, P.A.,
Darby, G.K. and Russell, N.J. eds). Fifty years of antimicrobials: Past Perspectives and
Future Trends. pp. 327 - 365. Cambridge University Press.
4) Regos, J. (1974). Investigations on the mode of action triclosan, a broad spectrum
antimicrobial. Zbl. Bakt. Hyg. I.Abt.Orig. A 226, 390-401.
 Back to Flow Cytometry and Microbiology Introductory Page
Back to Flow Cytometry and Microbiology Introductory Page





CD ROM Volume 4 was produced by staff at the Purdue University Cytometry Laboratories
and distributed free of charge as an educational service to the cytometry community.
If you have any comments please direct them to
Dr. J. Paul Robinson, Professor & Director, PUCL, Purdue University, West Lafayette,
IN 47907. Phone:(765) 494-0757; FAX (765) 494-0517; Web http://www.cyto.purdue.edu EMAIL cdrominfo@flowcyt.cyto.purdue.edu
 email: Robert_Jepras-1@sbphrd.com
email: Robert_Jepras-1@sbphrd.com

 email: Robert_Jepras-1@sbphrd.com
email: Robert_Jepras-1@sbphrd.com




 Back to Flow Cytometry and Microbiology Introductory Page
Back to Flow Cytometry and Microbiology Introductory Page