Ring Trials Lymphocyte Immunephenotyping
External Quality Control Survey of Immunephenotyping of the
Central Reference Institution in Germany
Issues of Specimen and Interlaboratory Variability
Organizers:
Working group of quality assessment in flow cytometry
of the German Society of Clinical Chemistry
Members:
W.Prohaska, R.Kruse, W.-J.Geilenkeuser,
T.Nebe, A.Radbruch, H.Diem, A.von Rücker
Address:
Deutsche Gesellschaft für Klinische Chemie e.V.
Zentrale Referenzinstitution, P.O.B 150 139, D-53040 Bonn, Germany,
Fax: +49/228/211529
Aim of the Survey:
Assessment and improvement of the interlaboratory
standardization for the determination of lymphocyte subpopulations as
defined by the markers CD3+, CD4+/CD3+, CD8+/CD3+, CD19+, CD16,56+/CD3- and
CD3+/HLA-DR+
Specimens:
In the first two surveys washed and purified buffy
coat, afterwards CDP (citrate/phosphate/dextrose) whole blood was
used. The buffy coat was magnetically immunodepleted (Milteny Biotec immuno
magnetic beads) to obtain pathologically low subpopulations. All specimens
were shipped on the day of blood drawing and cell preparation.
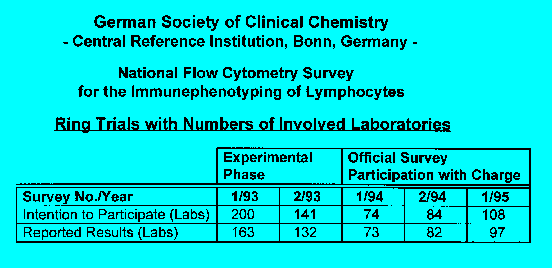
Summary of Results:
On average, 109 laboratories participated in the
surveys. After changing from immunodepleted buffy coat to CDP whole blood.
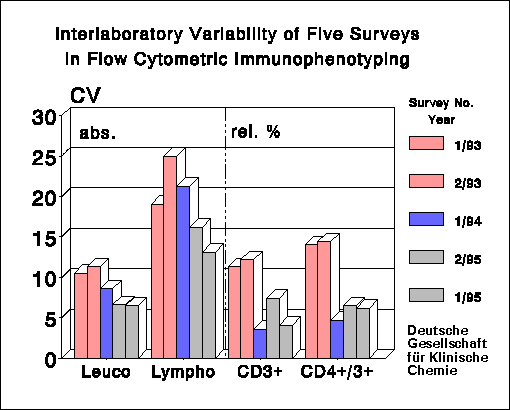
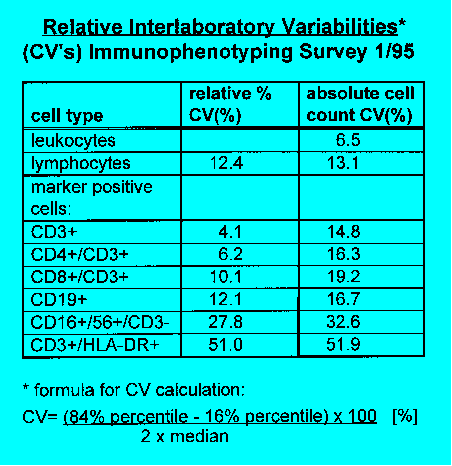
there was a distinct decrease of the coefficient of variation (CV) in the
determination of percentages of lymphocyte subpopulations: CV's improved
from 4.5-12% to 3-7.5% (CD3+ cells) and CV's of CD4+/CD3+ cells moved
from 5.8-14.4% to 3.2-6.6%. The CV's are lower than those reported by
the College of American Pathologists Flow Cytometry Survey (1) and by surveys
from other countries.
Clinically, the absolute cell count is more important than the percentages
which show relatively small CV's. For the absolute cell counts we calculated
CV's of 14-22.4% and 14.5-21% for CD3+ cells and CD4+/CD3+ cells, respectively.
These values are considerably worse than those for the corresponding percentage
counts.
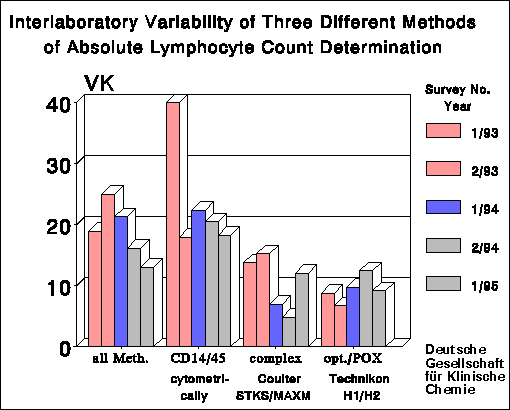
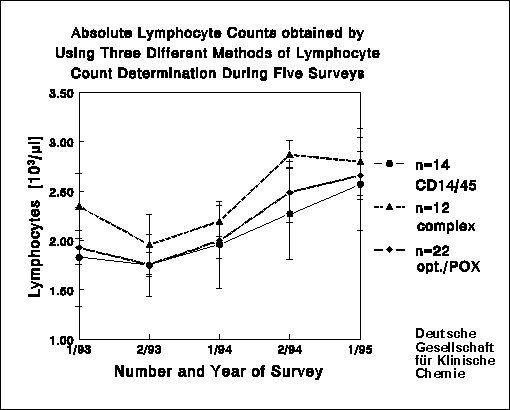
As the leukocyte and lymphocyte count is the basis for calculating of the
absolute cell counts of subpopulations, the calculation of these entities
is important and can raise CV's two- to threefold. Subpopulations with
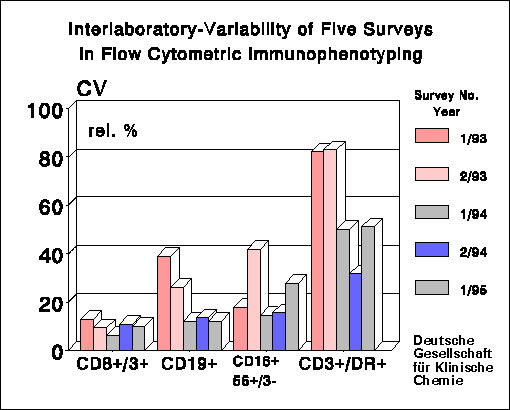
low percentages and/or inhomogenic expression of marker epitopes (for
instance HLA-DR) also yielded high CV's in relative counting.
Conclusion:
Fresh CPD whole blood is a natural, sufficiently
stable and easy to handle specimen material for quality surveys in
immunephenotyping. Aims to improve the results of quantitative immunephenotyping
should address white cell and lymphocyte counting. Further standardisation
of flow cytometry methods should improve quantitative immunophenotyping.
References:
1. Homburger, H.A., Rosenstock, W., Paxton, H., Paton, M.L., Landay, A.L.:
Assessment of Interlaboratory ariability of Immunephenotyping; Ann.NY Acad.
Sci.677:43-49(1993)
Images
Tab.1
Tab.2
For problems or comments, please contact:
G.Valet E-mail:
valet@vms.biochem.mpg.de,
Max-Planck-Institut für Biochemie,
Am Klopferspitz 18a, D-82152 Martinsried, Germany,
Tel: +49/89/8578-2518, -2525, Fax: +49/89/8578-2563,
INTERNET address: http://www.biochem.mpg.de/valet/cytorel.html
Last Update: Sep.20, 1996











