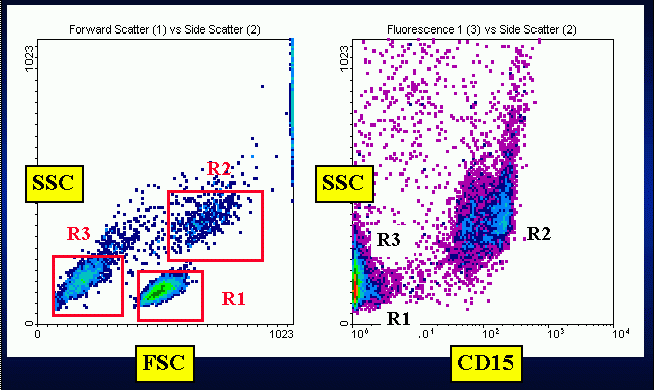
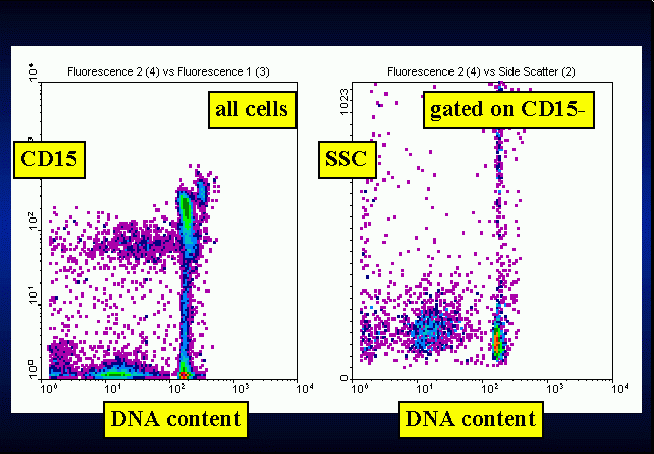
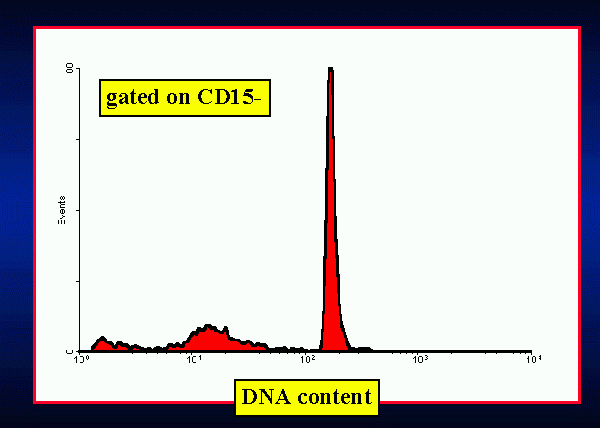
Briefly, this method consists of a first level, low cost and easy to
perform technique able to explore the susceptibility to PCD of whole blood
PBMC.
Instead of removing granulocytes and red blood cells by centrifugation
through density gradient, this technique focuses the DNA analysis on a
multiparametric defined cell population. Its rationale is based on a widely
accepted point, namely that the extrusion of apoptotic bodies gives the
apoptotic cell a hypodiploid content of DNA. Its main clinical application
could be the exploration of susceptibiliy to apoptosis in PBMC from patients
with autoimmune or viral diseases; nonetheless, an application in the evaluation
of peripheral blast response to anticancer drugs is possible.
This technique can be easily improved in a dual laser system using a
red-excitable dye (e.g. ToPro3) for DNA analysis, and introducing more
blu-excitable phenotypic or metabolic probes for further characterization
of subsets interested by the apoptotic process. Such modifications are
currently in development in our Laboratory.
Lower FCS concentration can result in an increase of debris, making
difficult the isolation of lymphocyte cluster on physical parameters.
In a group of 40 healthy persons (blood donors), the mean apoptotic
index was 0.04 (range 0.01-0.09) at 24 h, and 0,06 (range 0.03-0.15) at
48 h. A significative linear correlation (r=0.62) was found between values
measured at 24 and at 48 h (data not shown).
This method is very simple and fast. The time needed for the setting
up of cultures is negligible, and the time requested by sample staining
and analysis is similar to an ordinary immunophenotypic FACS analysis.
Moreover, when resuspended in ethanol, the sample can be stored for several
days at +4°C without perceptible loss of fluorescence.
1) Carbonari, M., M. Cibati, M. Cherchi, D. Sbarigia, A. M. Pesce,
L. Dell'Anna, A. Modica, and M. Fiorilli. 1994. Detection and characterization
of apoptotic peripheral blood lymphocytes in Human Immunodeficiency Virus
infection and cancer chemotherapy by a novel flow immunocytometric method.
Blood. 83:1268-1277.
2) Cossarizza, A., C. Mussini, N. Mongiardo, V. Borghi, G. Kalachnikova,
B. De Rienzo, and C. Franceschi. 1997. Mitochondria alterations and dramatic
tendency to undergo apoptosis in peripheral blood lymphocytes during acute
HIV syndrome. AIDS. 11:19-26.
3) Emlen, W., J. A. Niebur, and R. Kadera. 1994 Accelerated in vitro
apoptosis of lymphocytes from patients with systemic lupus erythematosus.
J. Immunol. 152:3685:3692.
4) Endresen, P. C., P. S. Prytz, and J. Aarbakke. 1995. A new flow cytometric
method for discrimination of apoptotic cells and detection of their cell
cycle specificity through staining of F-actin and DNA. Cytometry. 20:162-171.
5) Gougeon, M.-L., H. Lecoeur, A. Dulioust, M.-G. Enouf, M. Crouvoisier,
C. Goujard, T. Debord, and L. Montagnier. 1996. Programmed cell death in
peripheral lymphocytes from HIV-infected persons. Increased susceptibility
to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation
and with disease progression. J. Immunol. 156:3509-3520.
6) Jaleco, A. C., M. J. Covas, and R. M. M. Victorino. 1994. Analysis
of lymphocyte cell death and apoptosis in HIV-2-infected patients. Clin.
Exp. Immunol. 98:185-189.
7) Lorenz, H.-M., M. Grünke, T. Hieronymus, M. Herrman, A. Kühnel,
B. Manger, and J. R. Kalden. 1997. In vitro apoptosis and expression of
apoptosis-related molecules in lymphocytes from patients with systemic
lupus erythematosus and other autoimmune diseases. Arthritis Rheum.
40:306-317.
8) McCloskey, T. W., N. Oyaizu, M. Coronesi, and S. Pahwa. 1994. Use
of a flow cytometric assay to quantitate apoptosis in human lymphocytes.
Clin. Immunol. Immunopathol. 71:14-18.
9) Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C.
Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis
by propidium iodide staining and flow cytometry. J. Immunol. Methods.
139:271-279.
10) Ormerod, M. G., F. Paul, M. Cheetham, and X. M. Sun. 1995 Discrimination
of apoptotic thymocytes by forward light scatter. Cytometry. 21:300-304.
11) Pandolfi, F., M. Pierdominici, A. Oliva, G. D'Offizi, I. Mezzaroma,
B. Mollicone, A. Giovannetti, L. Rainaldi, I. Quinti, and F. Aiuti. 1995.
Apoptosis-related mortality in vitro of mononuclear cells from patients
with Hiv infection correlates with disease severity and progression. J.
Acquir. Immune. Defic. Syndr. 9:450-458.